PRC Research Projects
Ongoing PRC Projects
The PRC is involved in a variety of national and international multicentre studies. Below you can browse all of them, and find contact information to PI's and study coordinators. Several of the studies are recruiting centres. If your centre is interested in joining, please contact the person stated in the project information site.
MyPath is a 5 year research and innovation project funded by EU Horizon Europe. MyPath is led by Prof. Stein Kaasa from PRC at the Oslo University Hospital’s Department of Oncology, and Marie Fallon, head of the Palliative and Supportive Care group at the University of Edinburgh. This pan-European consortium involving clinicians, researchers, companies, and patient and professional associations will co-create digital patient-centred care pathways and implement them in nine cancer centres in eight European countries. Read more about MyPath here.
Main contact: Stein Kaasa

The 2-year Joint Action on Networks of Expertise (JANE) was initiated in October 2022, financed as part the EU4Health Program. JANE, coordinated by Istituto Nazionale dei Tumori (Italy) and supported by 34 other partners, is endorsed by the EU Commission, and aligns with the EU’s Beating Cancer Plan to improve the diagnosis and treatment of cancer patients across Europe.
The overall JANE goal was to shape seven new Networks of Expertise (NoE) in cancer field, organised as work packages (WP) in the following domains: personalized primary prevention; survivorship; palliative care; omic technologies; hi-tech medical resources; poor-prognosis cancer(s) and young adults with cancer. Five transversal task forces focus on issues important to cancer-related networking in the EU today. More explicitly, the objectives were to prepare everything necessary to launch the new NoEs, and to critically evaluate existing models of current and future EU networking with a view to optimize the functioning of the new NoEs and provide better access to health care services.
Oslo University Hospital has led the WP in Palliative Care. Our proof of concept was based on the Lancet Oncology Commission (Integration of oncology and palliative care: a Lancet Oncology Commission - ScienceDirect) to make PC part of routine cancer care in Europe. Five working groups were established focusing on basic elements to achieve this: defining the content of PC, indicators of PC, implementation, patient-centred pathways, and education/competence. Participants in the WGs come from 10 European countries besides Norway: Denmark, Estonia, Finland, Germany, Italy, Portugal, Romania, Spain, Sweden, and UK. They have provided important contributions and valuable considerations to the WP6 work regarding the diversities in health care organisation in general and oncology/palliative care provision in particular in Europe.
The final JANE report was presented to the EU parliament on Sept. 25th.
The call for a four-year extension of JANE was launched in 2023. The application proved successful, with the official start, November 1, 2024. JANE2 project focuses on sustainability, coordination between NoEs, information-technology infrastructure (including AI), and patient involvement. JANE2 and has 7 clinically oriented WPs and 3 that concern more ad ministrative and overarching themes, e.g. sustainability, evaluation, and dissemination. Read more about JANE2 here: JANE2 - Oslo universitetssykehus HF
Shaping the EU Networks of Expertise on cancer.
Main contacts: Stein Kaasa, Marianne Jensen Hjermstad

Hjernemetastasestudien.
Ongoing study
Patient inclusion closed March 2021, closure of follow-up by the end of 2022.
Background and study rationale
The overall BrainMet (BM) study comprises several subprojects, the prospective study presented below, supplemented with one large retrospective study of previous BM patients treated at Oslo University Hospital, one translational study on biomarkers and molecular similarities/dissimilarities of BMs and primary tumor tissue, and one qualitative study on patients' and caregivers' perception of how a BM diagnosis influences daily life and functioning from diagnosis and onwards. In Oct 2021, we also embarked on a follow-up study of long-term survivors of BM, consisting of thorough clinical and imaging examinations, self-reported QoL and physical and cognitive functioning and neurocognitive testing.
Patients diagnosed with brain metastases (BM) comprise a very heterogeneous group, with different cancer diagnoses, diverse symptoms and problems, treatment options and outcomes. The treatment offered varies across regions, and no systematic prospective follow-up has been conducted in Norway hitherto. The aim of this prospective BM-study, representing a Norwegian population-based cohort, is to follow patients from initial BM diagnosis during the disease and treatment trajectory with clinical data and patient-reported outcome measures.
Outcomes
Primary outcome is overall survival, treatment patterns and progression and QoL. Secondary outcomes are patient reported outcomes.
Methods
The BrainMet study is a multicenter, population-based prospective cohort study in cancer patients with newly diagnosed first BM. Patients are approached when admitted with a verified BM. Clinical data are registered every 3 months for 2 years, and consenting patients receive a set of PROMs by postal mail every month for at least 1 year or until death.
Results
Preliminary data show that more than 900 patients have been included. The median overall survival is approximately 6 months. Many patients die <3months after BM diagnosis, but >20% live >12 months. Almost 50% of patients treated with whole brain radiotherapy die < 3 months after diagnosis.
Clinical and research implications
The short life expectancy after the BM diagnosis emphasizes the importance of selecting the right treatment to the right patients, i.e. those who are most likely to benefit with the least impairments of QoL and functioning. Our findings so far suggest that a more restrictive use of whole brain radiotherapy should be considered. A closer follow-up of long-term survivors, i.e. patients living >1 year after diagnosis is necessary.
Publications
Winther RR et al. Surgery for brain metastases - real-world prognostic factors' association with survival. Acta Oncol 2021; https://doi.org/10.1080/0284186x.2021.1930150
Karlsson AT et al. Overall survival after initial radiotherapy for brain metastases; a population based study of 2140 patients with non-small cell lung cancer. Acta Oncol 2021; DOI: https://doi.org/10.1080/0284186x.2021.1924399
Gullhaug A et al. Use of radiotherapy in breast cancer patients with brain metastases– a retrospective 11-year single center study. J Med Imaging Radiat Sci 2021; DOI: https://doi.org/10.1016/j.jmir.2021.01.002
Main contact: Olav E. Yri
Computerized assessment of patient reported outcomes in cancer clinical care.
Closed project
The Eir development and implementation project was initiated about a decade ago, with the first computerized solution (PAT-C) developed by our group in Trondheim together with NTNU Technology Transfer AS (ntnutto.no). With EirV3, our work aims at widespread clinical use nationally and internationally in the next few years.
Background and study rationale
Several publications have addressed substantial shortcomings in systematic assessment and use of patient reported outcomes (PROMs) in cancer care. This is despite robust evidence of improved symptom management, QoL, and satisfaction with care, even prolonged survival. Eliciting the patient voice is instrumental in patient centered care; a major PRC research objective.
EirV3 is a user-friendly electronic solution that combines PROMs and treatment decision support, for use before and during the consultations, as well as remotely by patients and health care providers. Eir has been used in a clinical trial; COMBAT, at the cancer outpatient clinic at Trondheim University Hospital, and is currently part of the Norwegian cluster-RCT PALLiON. Usability results have shown that patients and health care providers found Eir intuitive, easy to use and relevant.
Outcomes
Primary project goals are to revise EirV3, extend the decision support section and to establish the necessary technical solutions compliant to all confidentiality and security regulations for implementation at OUH. Secondary goal is to promote widespread use in Norwegian cancer clinics as part of the medical health record systems. The long-term goal is to implement EirV3 as the standard symptom assessment tool in our international PRC-studies.
Methods
EirV3 has two modules: Eir-Patient for PROMs registration on tablets, and Eir-Doctor for presentation of PROMs in a user-friendly interface on computers. Eir-Patient starts with Level 0; screening of 19 common cancer symptoms. The endorsed symptoms are subject to scoring of intensity at Level 1, followed by symptom characterizations and in-depth questions at Level 2. The idea is to perform a systematic hierarchical symptom assessment from the patient's perspective. The pain section includes a body map for pain location and intensity. Questions about physical functioning, wellbeing are standard questions for all as are those on appetite and food intake for prediction of nutritional risk.
The patient's responses are immediately available wirelessly in Eir-Doctor and presented with a graphical overview of symptom development over time and the current symptom intensity scores. Symptoms with intensity scores >3 (0-10 scale) are marked in red, with brighter colors with higher intensities, supplemented with graphs displaying. At present, algorithms combining clinical information and PROMs provide decision support according to internationally acknowledged treatment guidelines for a few symptoms.
Clinical and research implications
The immediate implications is to perform a slight update of the content based on its use in PALLiON, and to establish the necessary technical solutions that allow widespread use in Norwegian Hospitals, according to all confidentiality and security regulations. A close collaboration with Imatis/DNV; a leading provider of ICT tools in health care, has been established to implement Eir at OUS to begin with. As EirV3 is one of the bearing elements of our recently submitted EU Horizon 2022 grant proposal, expansion is a long-term goal.
Publications
- Krogstad H et al. Computer-based symptom assessment is feasible in patients with advanced cancer: results from an international multicenter study, the EPCRC-CSA. J Pain Sympt Manage 2012; https://doi.org/10.1016/j.jpainsymman.2011.10.025
- Krogstad H et al. Development of EirV3: A Computer-Based Tool for Patient-Reported Outcome Measures in Cancer. JCO Clin Cancer Inform 2017; https://doi.org/10.1200/cci.17.00051
- Krogstad H et al. The paper on usability perceptions among end-users. Usability testing of EirV3-a computer-based tool for patient-reported outcome measures in cancer. Supp Care Cancer 2019; https://doi.org/10.1007/s00520-018-4435-3
- Raj SX et al. The COMBAT paper using Eir for pain assessment in an outpatient setting. COMBAT study - Computer based assessment and treatment - A clinical trial evaluating impact of a computerized clinical decision support tool on pain in cancer patients. Scand J Pain 2017; https://doi.org/10.1016/j.sjpain.2017.07.016
Main contacts: Stein Kaasa, Marianne Jensen Hjermstad
Paracetamol with Strong Opioids.
A randomized, double-blind, parallel-group non-inferiority phase III withdrawal trial of paracetamol versus placebo in conjunction with opioids for moderate to severe cancer-related pain.
Ongoing study
Patient inclusion, first patient included in October 2021
Background and study rationale
The World Health Organization's (WHO) three step analgesic ladder has been the standard approach for treating cancer-related pain for more than 30 years. However, this therapy is partly based on tradition and opinion and not on evidence from randomized trials.
Systematic reviews, including the Cochrane Review “Oral paracetamol (acetaminophen) for cancer pain" by Wiffen et al (2017), point out the lack of evidence of paracetamol's analgesic efficacy. Despite this, the prescribing of paracetamol in conjunction with strong opioids for patients with cancer pain is common practice in most countries worldwide and still recommended by the WHO.
There is a need to establish high quality evidence on whether paracetamol provides additional analgesic effect in cancer pain patients receiving strong opioids. Therefore, a definitive withdrawal study of paracetamol in patients using strong opioids for cancer pain will be an important contribution to provide evidence based care for patients with advanced cancer and reduce patients' burden from possible futile and inefficient treatment practices.
The primary study objective is to establish whether the analgesic efficacy of strong opioids is non-inferior after withdrawal of paracetamol compared to the analgesic efficacy of strong opioids and paracetamol for cancer-related pain.
Outcomes
The primary outcome is average pain intensity at day 8 measured by “Average pain past 24 hours" (numeric rating scale, NRS, 0-10). Secondary outcomes are changes in opioid requirements, opioid related side effects, and patient self-reported rating of overall improvement of pain.
Methods
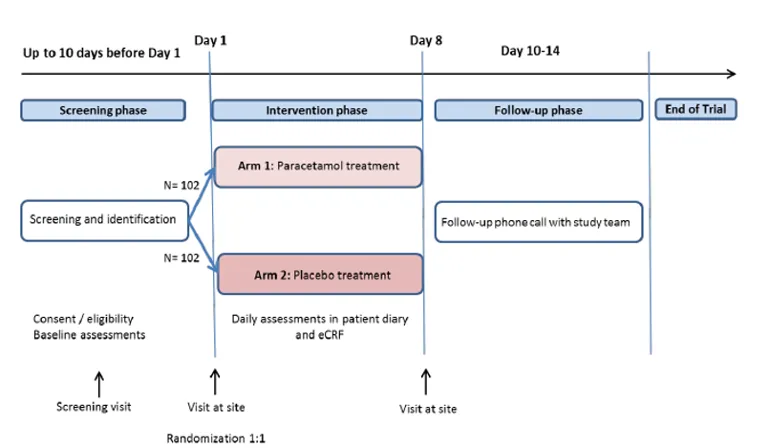
This is a prospective international multi-center parallel group, randomized, placebo-controlled, double-blinded non-inferiority withdrawal trial of placebo versus paracetamol in patients using strong opioids for cancer pain. The study will be conducted at 11 sites in Norway, UK and Italy. The inclusion period is estimated to about 24 months with last patient last visit in Q4 2023. A total number of 204 patients will be included.
Eligible patients have metastatic cancer with a life expectancy of more than 2 months; using both strong opioids and 1 gram of paracetamol 3-4 times daily. When entering the trial at baseline, Day 1, they will be randomized to receive either paracetamol or placebo for seven days in addition to their usual opioid doses. The study team will provide paracetamol or placebo tablets from day 1 of study. After a week, the participants will come to the last study visit (day 8). A follow-up phone call will be conducted 3-7 days after the day 8 visit.
Results
First results are anticipated early 2024.
Main contact: Ørnulf Paulsen
Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia trial.
Background and study rationale
Cancer cachexia is a multifactorial syndrome characterized by an ongoing loss of skeletal muscle mass that cannot be fully reversed by conventional nutritional support alone. Cachexia has a high prevalence in cancer and a major impact on patient physical function, morbidity and mortality. Despite the consequences of cachexia, there is no licensed treatment for cachexia and no accepted standard of care. It has been argued that the multifactorial genesis of cachexia lends itself to therapeutic targeting through a multimodal treatment approach. Following a successful phase II trial, a phase III randomized controlled trial was initiated. The MENAC intervention is based on evidence to date and consists of non-steroidal anti-inflammatory drugs and eicosapentaenoic acid to reduce inflammation, a physical exercise program using resistance and aerobic training to increase anabolism, combined with dietary counselling and oral nutritional supplements to promote energy and protein balance.
Outcomes
Study objective is to establish whether a multimodal intervention is effective in treating cachexia. The primary outcome is difference in body weight (Kg) at six weeks across groups. Secondary outcomes are difference in muscle mass (CT at L3) and physical activity (ActivPAL).
Methods
The MENAC trial is a multicenter randomized controlled trial of a multimodal therapy (ONS plus ibuprofen plus exercise) versus standard care. Following randomization, patients will be allocated to either the intervention or the control arm and key endpoints will be assessed after 6 weeks. Patients with NSCLC or pancreatic cancer starting palliative chemotherapy are eligible.
Clinical and research implications
At present, there are no established treatments for cachexia, and the condition is often neglected. Moreover, there is increasing evidence that current intensive oncological treatments are both exacerbating cachexia and are being curtailed by increased toxicity due to cachexia. There is consensus that cachexia is a multidimensional problem and that a multimodal approach to treatment is necessary. The intervention in the MENAC trial aims to establish a practical rehabilitation program that hopefully provide the first evidence-based method to reverse this currently intractable syndrome, that affects >50% of patients with advanced cancer and reduces their quality of life and life expectancy. Successful management of cachexia could have major impact on supportive oncology, but could in addition also improve the tolerance and probability of completing chemotherapy and radiotherapy treatment. This trial that includes patients from multiple countries, will hopefully help to improve the standardization of nutritional and metabolic care of patients undergoing anticancer therapy
Publications
Solheim, TS et al. Cancer cachexia: Rationale for the MENAC (Multimodal - Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Supportive & Palliative Care, 2018. DOI: https://doi.org/10.1136/bmjspcare-2017-001440
Solheim, TS et al. A randomized phase II trial of a multimodal intervention for the treatment of cachexia in lung and pancreatic cancer. J Cachexia Sarcopenia Muscle 2017. DOI: 10.1002/jcsm.12201
Main contacts: Marie Fallon, Stein Kaasa
Palliative radiotherapy in symptomatic pelvic soft tissue tumors (PallSoft)
Ongoing study
Patient inclusion started in December 2024. Estimated completed inclusion by December 2028, closure of follow-up December 2029.
Background and study rationale
Palliative radiotherapy is essential in the management of patients with symptomatic pelvic soft tissue tumors, providing rapid and efficient symptom relief. No standard treatment recommendations currently exist, yielding large differences in treatment regimen across cancer types and institutions.
Palliative patients have a limited life expectancy, which emphasizes the need for palliative radiotherapy to provide rapid symptom control within a limited overall treatment time. A short-course radiotherapy regimen with few radiotherapy fractions and high radiation dose per fraction is therefore favorable. A short-course radiotherapy regimen of 1-2 fractions has proven to be equally effective and tolerable as prolonged regimens in palliative patients with either painful bone metastases or thoracic symptoms. However, for patients with symptomatic pelvic soft tissue tumors in general, the optimal radiotherapy regimen is yet to be established
PallSoft is a national, randomized, phase III, non-inferiority study aiming to compare two short-course radiotherapy approaches.
Methods
The study will recruit 200 patients from 11 institutions over 2-4 years. Patients with symptomatic pelvic soft tissue tumor from gastrointestinal, urological or gynecological cancers candidates for palliative radiotherapy are eligible for inclusion. Patients will define their target symptom, and further be randomly assigned to receive palliative radiotherapy with either 1-2 fractions of 8 Gray (Gy) (arm A) or 5 fractions of 5 Gy (arm B).
Outcomes
The primary objective is to investigate whether patient-reported target symptom relief in arm A is non-inferior to arm B, assessed on a Numeric Rating Scale (NRS). Secondary outcomes are physician-assessed bowel/bladder toxicities and overall survival.
Explorative objectives include assessment of health-related quality of life, general patient satisfaction and health economic aspects. Prognostic models for survival prediction and predictive biomarkers for radiotherapy response will be explored.
Results
Currently no results
Clinical and research implications
The PallSoft study aims to address the current evidence gap regarding the most optimal radiotherapy regimen for palliative patients with symptomatic pelvic soft tissue tumors. The randomized and pragmatic study design, and the participation of all radiotherapy units in Norway, will provide data approximating a real-world situation, which in turn will enhance clinical applicability and facilitate implementation of study results. The focus on patient-centered and patient-reported endpoints will provide systematic and warranted data on important aspects in assessment of treatment benefit, including QoL. Prognostic and predictive biomarkers will present individualized data on expected treatment benefit, and may allow for future personalized treatment approaches.
Publications
Currently no publications
Clinicaltrials.gov identifier: NCT06398314,
Main contacts
Kjersti Skipar (national coordinating investigator), kjeski@sthf.no
Harald Bull Ragnum (project leader), harrag@sthf.no