Flecainide in arrhythmic mitral valve prolapse (FLECAPRO)
FLECAPRO is an investigator-initiated, prospective, randomized, open-label, blinded-endpoint, crossover study with the goal to assess the efficacy and safety of adding flecainide to standard beta-blocker therapy in patients with arrhythmic mitral valve prolapse. The primary endpoint is the number of ventricular tachyarrhythmias (severe and life-threatening arrhythmias) on implantable heart rhythm monitors during 12 months in each treatment arm.
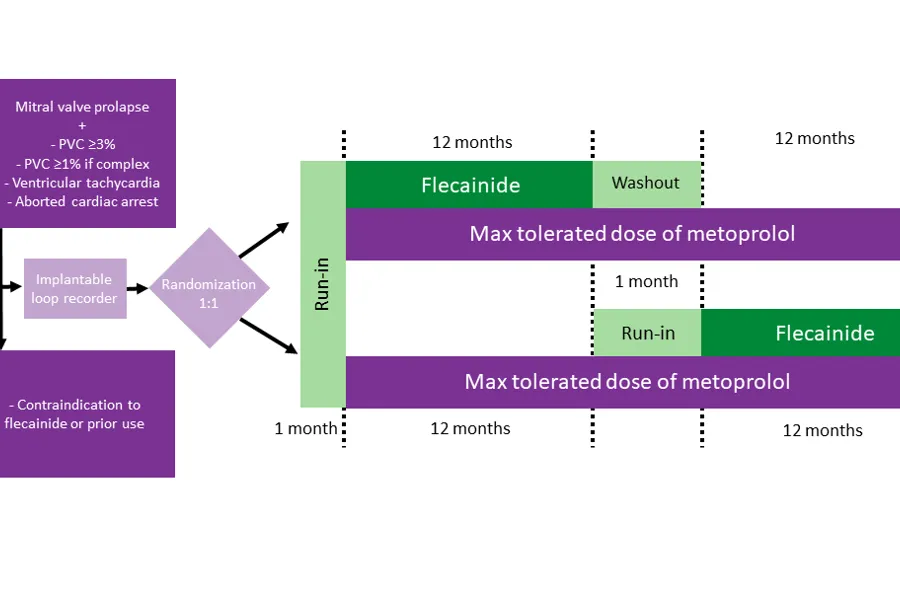
Secondary endpoints are the number of premature ventricular beats on 24-hour home monitors, patient-reported health-related quality of life, and the number of life-threatening ventricular tachyarrhythmias. Oslo University Hospital is the only centre, and St. Olavs Hospital provides blinded primary endpoint adjudication and analyses of flecainide serum concentrations. All participants will be monitored by implantable heart rhythm monitors, as well as through clinical visits, physical examinations, blood samples, ECG, Holter monitoring and echocardiography.
- You can find more information about the study in norwegian on the OUS website.
- Details about the study can also be found on ClinicalTrials.gov
Researchers involved
- Eivind W. Aabel, MD/PhD (principal investigator)
- Cecilie Bugge, MD/PhD fellow
- Christian Five, MD/PhD fellow
- Kristina Haugaa, MD/Professor